Ch. 2 Notes
Matter is anything that occupies space and has mass.
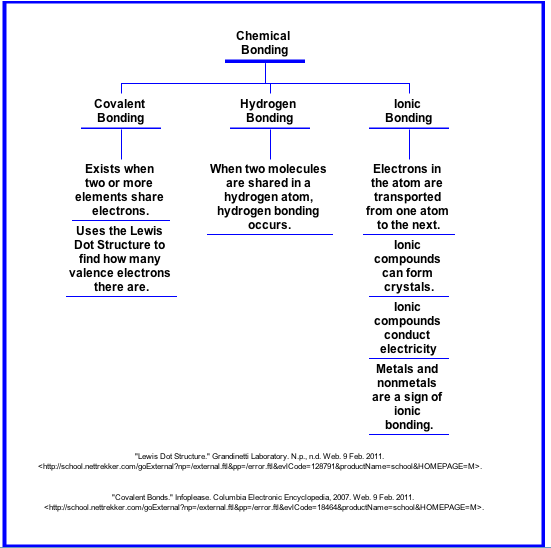
An element is a substance that cannot be broken down into other substances by chemical reactions.
There are 92 naturally occurring elements.
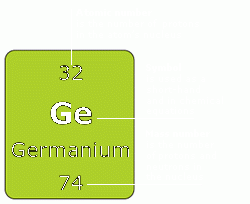

Trace elements make up less than 0.01% of a human's weight, but we cannot live without them.
An atom is the smallest unit of matter that still retains the properties of an element.
A proton has a positive charge.
An electron has a negative charge.
A neutron has no charge.
Nucleus - the atom's central core.
Mass is the measure of the amount of material in an object.
Isotope - Variant of an atom. Has different number of neutrons.
Radioactive isotope - nucleus decays spontaneously, giving off particles and energy