Life would not be possible without water.
Water sticks together because of cohesion.
Surface tension is how difficult it is to break the surface of a liquid.
Water moderates temperature.
Water can be used as a solvent.


Ch. 2 HW
The tendency of molecules of the same kind to stick together is called _________. cohesion
Physicians have used imaging techniques for medical diagnosis for some time, but recently have begun usage of magnetic resonance imaging (MRI), which depends on _______. the behavior of hydrogen atoms in water molecules in a strong magnetic field
If you were asked to determine if there is life on Mars, what would you propose to do? Look for water.
Carbon-12 and carbon-13 are different in the number of their _____. neutrons
The bond between the hydrogen atom of one water molecule and the oxygen atom of another water molecule is made possible by _____. the polarity of the water molecule
The simplest unit of matter that still retains the properties of an element is a(n) _____. atom
What type of bond joins neighboring water molecules? hydrogen
A goiter is an enlargement of the thyroid gland that is associated with abnormal thyroid function. The worldwide incidence of goiters is about 16%. Third-world countries are especially vulnerable to serious health problems related to goiters. What element is deficient in the diet of most of the people who have goiters? iodine
Which of the following is one of the four most abundant elements that make up the human body? carbon
The bond formed by the attraction of oppositely charged ions is a(n) _____ bond. ionic
Substances that cannot be broken down into other substances are called _____. elements
H2O is an example of a(n) _____. compound
Fluorine's atomic number is 9 and its mass number is 19. Which of the following is true of fluorine? It contains 9 protons and 10 neutrons.
What is the pH inside most living cells? pH 7
In the reaction glucose + fructose → sucrose + water, _____ is a reactant and _____ is a product. glucose ... water
Substances that cannot be broken down into other substances are called _____. elements
Which of the following describes a covalent bond? electron sharing
Substances that contain two or more elements in a fixed ratio are called _____. compounds
Isotopes of an element differ in their _____. mass number
Ch. 2 Quiz
Examine the drawing of an atom below. The art is technically incorrect in that ______.
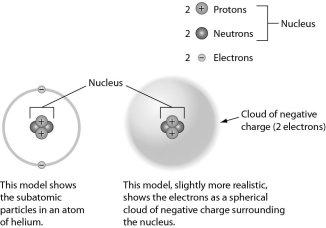
the electrons should be much farther away from the nucleus
People have long speculated about whether life exists on Mars. Scientists have evidence that on Mars, ______. liquid water has existed in the past
When a base is added to a buffered solution, the buffer will ______. donate H+ ions
As water freezes, ______. its molecules move farther apart
Sugar dissolves when stirred into water. The sugar is the ______, the water is the ______, and the sweetened water is the ______. solute... solvent... solution
Examine the following figure. Which of the representations of molecules does not reveal double bonds?
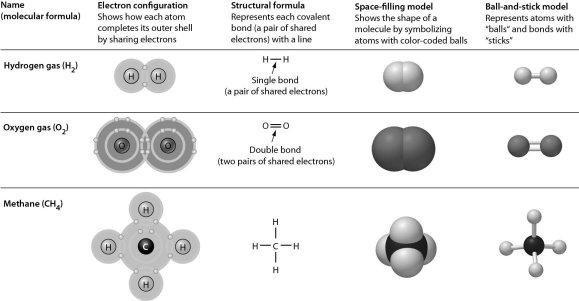
space-filling model
Which of the following is an acid? HCl
An atom with an electrical charge is a(n) ______. ion
The bond between oppositely charged ions is a(n) ______ bond. ionic
The four most common elements found in living things are carbon, oxygen, nitrogen, and hydrogen.

